 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| CDD-C52H8 | Cynomolgus | Cynomolgus CD3 delta Protein, His Tag |  |
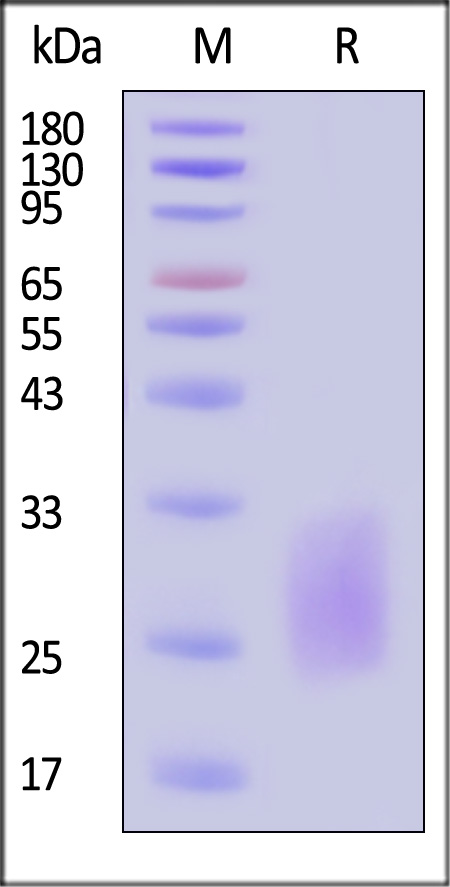
|
|
| CDD-H5224 | Human | Human CD3 delta Protein, His Tag |  |
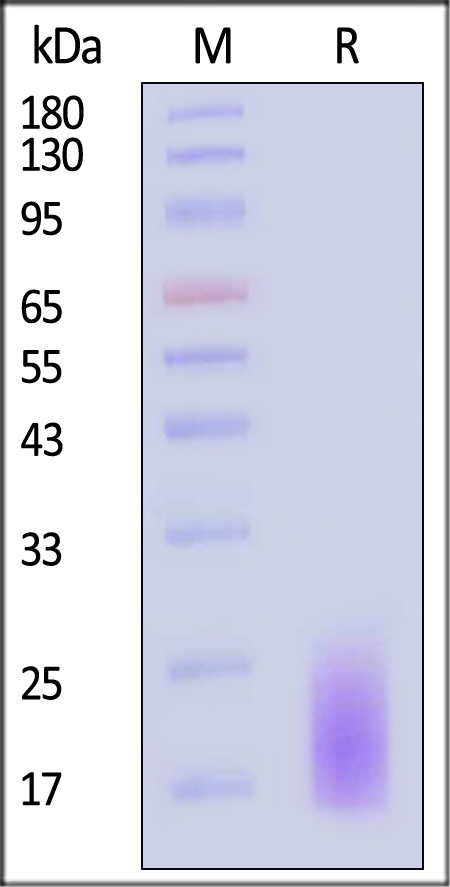
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse monoclonal antibody against human CD3 antigen of T lymphocyte (Wuhan Institute of Biological Products) | Approved | Wuhan Institute Of Biological Products Co Ltd | Mainland China | Rejection of organ transplantation | Wuhan Institute Of Biological Products Co Ltd | 1999-01-01 | Rejection of organ transplantation | Details | ||
| Tebentafusp | ImmTAC-gp-100; IMC-gp-100; IMC-gp100 | Approved | Immunocore Ltd | KIMMTRAK | United States | Uveal melanoma | Immunocore Ltd | 2022-01-25 | Uveal melanoma; Melanoma | Details |
| Teclistamab | JNJ-64007957; JNJ-64007959; JNJ-7957 | Approved | Johnson & Johnson Innovative Medicine, Genmab A/S | TECVAYLI, TECAYLI | EU | Multiple Myeloma | Janssen-Cilag International Nv | 2022-08-23 | Hematologic Diseases; Hematologic Neoplasms; Multiple Myeloma | Details |
| Teplizumab | MGA-031; PRV-031; hOKT3-γ1-ala-ala; hOKT3-gamma-1-ala-ala | Approved | Tolerance Therapeutics Inc | TZIELD | United States | Diabetes Mellitus, Type 1 | Provention Bio Inc | 2022-11-17 | Diabetes Mellitus, Type 1; Rejection of renal transplantation; Glucose Intolerance; Hypoglycemia; Psoriasis; Diabetes Mellitus, Experimental | Details |
| Mosunetuzumab | BTCT-4465A; RO-7030816; CD20-TBD; RG-7828 | Approved | Genentech Inc | Lunsumio | EU | Lymphoma, Follicular | Roche Registration Gmbh | 2022-06-03 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lupus Erythematosus, Systemic; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Epcoritamab | GEN-3013; ABBV-GMAB-3013 | Approved | Genmab A/S, Abbvie Inc | Tepkinly, TEPKINLY, EPKINLY | United States | Lymphoma, Large B-Cell, Diffuse | Genmab Us Inc | 2023-05-19 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Richter's Syndrome; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Blinatumomab | BiTE-MT-103; bscCD19xCD3; AMG-103; MT-103; MEDI-538 | Approved | Micromet Inc | Blincyto, 倍利妥 | United States | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Amgen Inc | 2014-12-03 | Leukemia; Leukemia, Myelogenous, Chronic; Neoplasm, Residual; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Multiple Myeloma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Philadelphia Chromosome; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Mouse monoclonal antibody against human CD3 antigen of T lymphocyte (Wuhan Institute of Biological Products) | Approved | Wuhan Institute Of Biological Products Co Ltd | Mainland China | Rejection of organ transplantation | Wuhan Institute Of Biological Products Co Ltd | 1999-01-01 | Rejection of organ transplantation | Details | ||
| Tebentafusp | ImmTAC-gp-100; IMC-gp-100; IMC-gp100 | Approved | Immunocore Ltd | KIMMTRAK | United States | Uveal melanoma | Immunocore Ltd | 2022-01-25 | Uveal melanoma; Melanoma | Details |
| Teclistamab | JNJ-64007957; JNJ-64007959; JNJ-7957 | Approved | Johnson & Johnson Innovative Medicine, Genmab A/S | TECVAYLI, TECAYLI | EU | Multiple Myeloma | Janssen-Cilag International Nv | 2022-08-23 | Hematologic Diseases; Hematologic Neoplasms; Multiple Myeloma | Details |
| Teplizumab | MGA-031; PRV-031; hOKT3-γ1-ala-ala; hOKT3-gamma-1-ala-ala | Approved | Tolerance Therapeutics Inc | TZIELD | United States | Diabetes Mellitus, Type 1 | Provention Bio Inc | 2022-11-17 | Diabetes Mellitus, Type 1; Rejection of renal transplantation; Glucose Intolerance; Hypoglycemia; Psoriasis; Diabetes Mellitus, Experimental | Details |
| Mosunetuzumab | BTCT-4465A; RO-7030816; CD20-TBD; RG-7828 | Approved | Genentech Inc | Lunsumio | EU | Lymphoma, Follicular | Roche Registration Gmbh | 2022-06-03 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lupus Erythematosus, Systemic; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Epcoritamab | GEN-3013; ABBV-GMAB-3013 | Approved | Genmab A/S, Abbvie Inc | Tepkinly, TEPKINLY, EPKINLY | United States | Lymphoma, Large B-Cell, Diffuse | Genmab Us Inc | 2023-05-19 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Richter's Syndrome; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Blinatumomab | BiTE-MT-103; bscCD19xCD3; AMG-103; MT-103; MEDI-538 | Approved | Micromet Inc | Blincyto, 倍利妥 | United States | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Amgen Inc | 2014-12-03 | Leukemia; Leukemia, Myelogenous, Chronic; Neoplasm, Residual; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Multiple Myeloma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Philadelphia Chromosome; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| M-701 | M-701 | Phase 3 Clinical | Wuhan Yzy Biopharma Co Ltd | Ovarian Neoplasms; Solid tumours; Hydrothorax; Stomach Neoplasms; Neoplasms; Ascites; Colorectal Neoplasms; Pleural Effusion, Malignant; Carcinoma, Non-Small-Cell Lung | Details |
| ABBV-383 | TNB-383B; ABBV-383 | Phase 3 Clinical | Teneobio Inc, Abbvie Inc | Multiple Myeloma | Details |
| IMC-F106C | IMC-F106C; PRAME HLA-A02 | Phase 3 Clinical | Immunocore Ltd | Solid tumours; Melanoma | Details |
| Alnuctamab | CC-93269; EM-901; BMS-986349 | Phase 3 Clinical | Engmab Ag | Multiple Myeloma | Details |
| Catumaxomab | LP000 | Phase 3 Clinical | Trion Research, Neovii Biotech Gmbh | Ovarian Neoplasms; Stomach Neoplasms; Carcinoma; Neoplasms; Carcinoma, Ovarian Epithelial; Colonic Neoplasms; Urinary Bladder Neoplasms; Ascites; Breast Neoplasms | Details |
| Flotetuzumab | S-80880; MGD-006; RES-234 | Phase 2 Clinical | Macrogenics Inc | Leukemia, Myelogenous, Chronic; Hematologic Neoplasms; Leukemia; Leukemia, Hairy Cell; Mastocytosis, Systemic; Hodgkin Disease; Blastic Plasmacytoid Dendritic Cell Neoplasm; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Neoplasms, Plasma Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Leukemia, Myeloid, Acute; Leukemia, Biphenotypic, Acute | Details |
| Anti-CD3/humanized 3F8 bispecific antibody-activated T lymphocytes (University of Virginia) | Phase 2 Clinical | University Of Virginia | Neuroblastoma | Details | |
| Imvotamab | IGM-2323 | Phase 2 Clinical | Igm Biosciences Inc | Lymphoma, B-Cell, Marginal Zone; Myositis; Lupus Erythematosus, Cutaneous; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Arthritis; Lymphoma, Non-Hodgkin | Details |
| Foralumab | TZLS-401; NI-0401/α-CD3; NI-0401 | Phase 2 Clinical | Novimmune Sa, Bristol-Myers Squibb Company | Respiratory Tract Infections; Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Coronavirus Disease 2019 (COVID-19); Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Alzheimer Disease; Crohn Disease | Details |
| GNR-084 | GNR-084 | Phase 2 Clinical | Generium Pharmaceuticals, Iontas | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD3 and anti-HER2 BiTE-expressing T cell (MedImmune) | Phase 2 Clinical | University Of Michigan, Huazhong University Of Science And Technology, University Of Virginia Cancer Center, Medimmune | Breast Neoplasms | Details | |
| Vibecotamab | XmAb-14045 | Phase 2 Clinical | Xencor Inc | Leukemia, Myeloid; Myelodysplastic Syndromes; Blastic Plasmacytoid Dendritic Cell Neoplasm; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details |
| α/β CD3+/CD19+ cell depleted stem cell therapy (Mitchell Cairo) | Phase 2 Clinical | New York Medical College | Leukemia; Anemia; Thalassemia; Hodgkin Disease; Anemia, Aplastic; Thrombocytopenia; Lymphoma, Non-Hodgkin; Kostmann Syndrome; Anemia, Sickle Cell | Details | |
| MK-6070 | HPN-328; MK-6070 | Phase 2 Clinical | Harpoon Therapeutics | Small Cell Lung Carcinoma | Details |
| Cibisatamab | CEA-TCB; RG-7802; RO-6958688; CEA-CD3 TCB | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CD3/CD19 neg allogeneic BMT (National Institute of Allergy and Infectious Diseases/University of Pittsburgh) | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), University Of Pittsburgh | Primary Immunodeficiency Diseases; Female Urogenital Diseases; Inflammation | Details | |
| HPN-536 | HPN-536 | Phase 2 Clinical | Harpoon Therapeutics | Neoplasms | Details |
| MBS-303 | MBS-303 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| HBM-7022 | HBM-7022; AZD-5863 | Phase 2 Clinical | Harbour Biomed | Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Neoplasms; Digestive System Neoplasms; Esophageal adenocarcinoma; Carcinoma, Pancreatic Ductal; Gastrointestinal Neoplasms | Details |
| TAK-280 | MVC-280; TAK-280 | Phase 2 Clinical | Maverick Therapeutics Inc | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| GB-261 | GB-261 | Phase 2 Clinical | Genor Biopharma Co Ltd | Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| REGN-5459 | REGN-5459 | Phase 2 Clinical | Multiple Myeloma; Renal Insufficiency, Chronic | Details | |
| IBI-3003 | IBI3003; IBI-3003 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Multiple Myeloma | Details |
| MBS-314 | MBS-314 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Multiple Myeloma | Details |
| RO7515629 | RO7515629; RO-7515629; RG-6353 | Phase 2 Clinical | F. Hoffmann-La Roche Ag | Ovarian Neoplasms; Carcinoma, Renal Cell; Pancreatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BI-764532 | BI-764532; OBT620 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Small Cell Lung Carcinoma; Neoplasms; Neuroendocrine Tumors; Carcinoma, Neuroendocrine; Glioma | Details |
| GEN3017 | GEN-3017 | Phase 2 Clinical | Genmab A/S | Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| MP0533 | MP0533 | Phase 2 Clinical | Molecular Partners Ag | Leukemia, Myeloid, Acute | Details |
| CD30 biAb-AATC(The Medical College Of Wisconsin Nonprofit) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Leukemia; Hodgkin Disease; Lymphoma; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell, Cutaneous | Details | |
| APVO-436 | APVO-436; APVO436 | Phase 2 Clinical | Aptevo | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| Emfizatamab | GNC-038 | Phase 2 Clinical | SystImmune | Solid tumours; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Central Nervous System Lymphoma | Details |
| GEN-1047 | GEN-1047 | Phase 2 Clinical | Genmab A/S | Carcinoma, Non-Small-Cell Lung | Details |
| REGN-4336 | REGN-4336 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant | Details | |
| CM-350 | CM-350 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Solid tumours | Details |
| 1-A-46 | BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 | Phase 2 Clinical | Chengdu Chimagen Biosciences Co Ltd, BioRay Pharmaceutical Co Ltd | Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| LBL-034 | LBL-034 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms; Multiple Myeloma | Details |
| LBL-033 | LBL-033 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms | Details |
| CM-336(Connaught Biomedical Technology) | CM-336 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Multiple Myeloma | Details |
| ICP-B02 | CM-355; ICP-B02 | Phase 2 Clinical | Beijing Tiannuo Jiancheng Pharmaceutical Technology Co Ltd, Keymed Biosciences Co Ltd | Hematologic Neoplasms; Hematoma; Lymphoma; Lymphoma, Non-Hodgkin | Details |
| SMET-12 | SMET-12 | Phase 2 Clinical | Zhejiang Shimai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| QLS-31905 | QLS-31905 | Phase 2 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| Nebratamig | GNC-035 | Phase 2 Clinical | Solid tumours; Hematologic Neoplasms; Breast Neoplasms; Metastatic breast cancer; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| TAK-186 | EGFR x CD3 COBRA; MVC-101; TAK-186 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| EMB-06 | EMB-06; EMB06 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Multiple Myeloma | Details |
| AZD-0486 | TNB-486; AZD-0486; AZD0486 | Phase 2 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| BNT-142 | BNT-142 | Phase 2 Clinical | Biontech Se | Solid tumours | Details |
| CN-201 | CN-201 | Phase 2 Clinical | Curon Biopharmaceutical Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| ZG006 | ZG006; ZG-006 | Phase 2 Clinical | Gensun Biopharma Inc, Suzhou Zelgen Biopharmaceuticals Co Ltd | Solid tumours; Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Neuroendocrine | Details |
| EX-103 | EX-103; EX103 | Phase 2 Clinical | Guangzhou Excelmab Inc | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD3 monoclonal antibody (Jinan Tiankang Biological Products) | Phase 2 Clinical | Jinan Tiankang Biological Products Co Ltd | Anemia, Aplastic | Details | |
| Cevostamab | RG-6160; BFCR-4350A; RO-7187797 | Phase 2 Clinical | Genentech Inc | Multiple Myeloma | Details |
| Anti-CD3/anti-EGFR -activated T cells (Barbara Ann Karmanos Cancer Institute) | Phase 2 Clinical | Barbara Ann Karmanos Cancer Institute, University Of Virginia | Glioblastoma; Pancreatic Neoplasms | Details | |
| Ubamatamab | REGN-4018 | Phase 2 Clinical | Ovarian Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms | Details | |
| Anti-CD3-anti-HER2-activated T cells | Phase 2 Clinical | Transtarget | Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Prostatic Neoplasms | Details | |
| JCAR-014 | JCAR-014; JCAR021 | Phase 2 Clinical | Fred Hutchinson Cancer Research Center, Memorial Sloan Kettering Cancer Center, Seattle Children'S Research Institute, Juno Therapeutics Inc | Lymphoma, B-Cell; Leukemia; Leukemia, Lymphoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Candidiasis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| A-319 | A-319 | Phase 1 Clinical | Evive Biotech Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| BCMA TriTAC | HPN-217 | Phase 1 Clinical | Harpoon Therapeutics, Abbvie Inc | Multiple Myeloma | Details |
| Membrane bound ligand T-SIGn virus | NG-348 | Phase 1 Clinical | Akamis Bio Ltd | Neoplasms | Details |
| NG-641 | EnAd-FAP-BiTE; EnAd-FAP-Tac; NG-641; NG-aFAP | Phase 1 Clinical | Akamis Bio Ltd, University Of Oxford | Squamous Cell Carcinoma of Head and Neck; Neoplasms, Glandular and Epithelial; Neoplasm Metastasis | Details |
| Vixtimotamab | T-564; AMV-564 | Phase 1 Clinical | Amphivena Therapeutics Inc | Solid tumours; Myelodysplastic Syndromes | Details |
| MGD-014 | MGD-014 | Phase 1 Clinical | Macrogenics Inc | HIV Infections | Details |
| JNJ-63898081 | JNJ-8081; JNJ-63898081 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| K-193 | K-193 | Phase 1 Clinical | Beijing Lvzhu Biological Technology Co Ltd | Lymphoma, B-Cell | Details |
| Anti-EGFR-bispecific antibody armed activated T-cell therapy (Memorial Sloan Kettering Cancer Center) | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Pancreatic Neoplasms | Details | |
| Runimotamab | BTRC-4017A; RG-6194; RO-7227780 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| Autologous T-cell therapy (anti-PSMA-CD3), Roger Williams Medical Center | Phase 1 Clinical | Roger Williams Medical Center | Prostatic Neoplasms | Details | |
| ONO-4685 | ONO-4685 | Phase 1 Clinical | Merus Nv | Psoriasis; Lymphoma, T-Cell; Plaque psoriasis | Details |
| WVT-078(Novartis Pharma) | WVT-078 | Phase 1 Clinical | Novartis Pharma Ag | Multiple Myeloma | Details |
| M-802(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | M-802 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| Emirodatamab | AMG-427 | Phase 1 Clinical | Amgen Inc | Leukemia, Myeloid, Acute | Details |
| JNJ-63709178 | JNJ-9178; CNTO-9958; JNJ-63709178 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine, Genmab A/S | Leukemia, Myeloid, Acute | Details |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| CC-1 | CC-1 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Squamous Cell | Details |
| Acapatamab | AMG-160 | Phase 1 Clinical | Amgen Inc | Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| Pavurutamab | AMG-701 | Phase 1 Clinical | Amgen Inc | Multiple Myeloma | Details |
| Xaluritamig | AMG-509 | Phase 1 Clinical | Xencor Inc, Amgen Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| CC-3 | CC-3 | Phase 1 Clinical | Eberhard Karls University Of Tubingen, Germany | Colorectal Neoplasms; Gastrointestinal Neoplasms | Details |
| JS-203 | JS-203 | Phase 1 Clinical | Shanghai Junshi Biological Engineering Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| BC-004 | BC-004 | Phase 1 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Breast Neoplasms | Details |
| JY-016 | JY-016 | Phase 1 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Solid tumours; Colorectal Neoplasms; Lung Neoplasms | Details |
| EX-105 | EX-105; EX105 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| EMB-07 | EMB-07 | Phase 1 Clinical | Shanghai Epimab Biotherapeutics, Inc | Solid tumours; Ovarian Neoplasms; Stomach Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Adenocarcinoma of Lung; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Lymphoma; Uterine Neoplasms; Neoplasm Metastasis | Details |
| BA-3182 | BA-3182 | Phase 1 Clinical | Bioatla | Solid tumours; Adenocarcinoma | Details |
| BA-1202 | BA-1202 | Phase 1 Clinical | Solid tumours | Details | |
| CLN-978 | CLN-978 | Phase 1 Clinical | Adimab LLC | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| MGD-024 | MGD-024 | Phase 1 Clinical | Macrogenics Inc | Leukemia, Myelogenous, Chronic; Leukemia, Hairy Cell; Hodgkin Disease; Myelodysplastic Syndromes; Neoplasms; Blastic Plasmacytoid Dendritic Cell Neoplasm; Leukemia, B-Cell; Leukemia, Myeloid, Acute | Details |
| ARB-202 | ARB-202 | Phase 1 Clinical | Arbele Corp | Liver Neoplasms; Biliary Tract Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Esophageal adenocarcinoma; Gastrointestinal Neoplasms | Details |
| CX-904 | CX-904 | Phase 1 Clinical | Amgen Inc, Cytomx Therapeutics Inc | Solid tumours; Neoplasms | Details |
| CLN-049 | CLN-049 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| XmAb-819 | XmAb-819 | Phase 1 Clinical | Xencor Inc | Kidney Neoplasms; Carcinoma, Renal Cell | Details |
| JNJ-70218902 | JNJ-70218902 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| JNJ-67571244 | JNJ-67571244; JNJ-67371244; JNJ-1244 | Phase 1 Clinical | Johnson & Johnson | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| SCTB-35 | SCTB35; SCTB-35 | Phase 1 Clinical | SinoCelltech Ltd | Lymphoma, B-Cell | Details |
| JNJ-87890387 | JNJ-87890387 | Phase 1 Clinical | Janssen Research & Development Llc | Solid tumours | Details |
| XmAb-541 | XmAb541; XmAb-541 | Phase 1 Clinical | Xencor Inc | Ovarian Neoplasms; Ovarian germ cell tumor; Germinoma; Endometrial Neoplasms; Neoplasms, Germ Cell and Embryonal | Details |
| [89Zr]Zr-BI-764532 | Phase 1 Clinical | C.H. Boehringer Sohn Ag & Co. Kg, Boehringer Ingelheim Gmbh | Small Cell Lung Carcinoma; Carcinoma, Neuroendocrine | Details | |
| TGI-6 | TGI-6 | Phase 1 Clinical | Hefei TG ImmunoPharma Co Ltd | Solid tumours; Colorectal Neoplasms | Details |
| SIM-0500 | SIM-0500; SIM0500 | Phase 1 Clinical | Hainan Xiansheng Re Ming Pharmaceutical Co Ltd | Bone Marrow Neoplasms; Multiple Myeloma | Details |
| Recombinant anti BCMA/CD3 bispecific antibody(Hualan Genetic Engineering) | Phase 1 Clinical | Hualan Genetic Engineering (Henan) Co Ltd | Multiple Myeloma | Details | |
| Oncolytic Virus R130(Yunying Medical) | R-130-OV; R130; R-130 | Phase 1 Clinical | Shanghai Yunying Medical Technology Co Ltd | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Melanoma; Laryngeal Neoplasms; Pharyngeal Neoplasms; Lung Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Bone Neoplasms; Peritoneal Neoplasms; Sarcoma; Osteosarcoma; Head and Neck Neoplasms; Brain Neoplasms; Otorhinolaryngologic Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Nose Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma; Carcinoma, Bronchogenic; Liver Neoplasms; Ovarian Neoplasms; Kidney Neoplasms | Details |
| ASP2074 | ASP-2074; ASP2074 | Phase 1 Clinical | Astellas Pharma Global Development Inc | Solid tumours | Details |
| OBT620 | OBT620 | Phase 1 Clinical | Boehringer Ingelheim Gmbh, Oxford Biotherapeutics Ltd | Small Cell Lung Carcinoma | Details |
| CBA-1535 | CBA-1535 | Phase 1 Clinical | Chiome Bioscience Inc | Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Mesothelioma; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-008 | EGFR-TRACTr; JANX008; JANX-008 | Phase 1 Clinical | Janux Therapeutics Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-007 | JANX-007; PSMA-TRACTr | Phase 1 Clinical | Janux Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| TQB-2934 | TQB2934; TQB-2934 | Phase 1 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Multiple Myeloma | Details |
| JNJ-80948543 | JNJ-80948543 | Phase 1 Clinical | Janssen Research & Development Llc | Lymphoma, B-Cell; Neoplasms; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| PIT-565 | PIT-565 | Phase 1 Clinical | Novartis Pharma Ag | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Leukemia, Myeloid, Acute | Details |
| CC-312 | CC-312 | Phase 1 Clinical | CytoCares (Shanghai) Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ASP-2138 | ASP-2138 | Phase 1 Clinical | Astellas Pharma Global Development Inc, Xencor Inc | Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms | Details |
| ISB-2001 | ISB-2001 | Phase 1 Clinical | Ichnos Sciences Sa | Multiple Myeloma | Details |
| GR-1901 | GR1901; GR-1901 | Phase 1 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Leukemia, Myeloid, Acute | Details |
| AMG-794 | AMG-794 | Phase 1 Clinical | Amgen Inc | Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung | Details |
| QLS-31904 | QLS31904; QLS-31904 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours | Details |
| YK-012 | YK012 | Phase 1 Clinical | Lymphoma, B-Cell | Details | |
| RO-7428731 | RO-7428731; RG-6156 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Glioblastoma | Details |
| GBR-1342 | GBR-1342; ISB-1342 | Phase 1 Clinical | Glenmark Pharmaceuticals Ltd | Multiple Myeloma | Details |
| Recombinant humanized anti-CD19/CD3 bispecific antibody(New Time Pharmaceutical) | LNF-1904 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| XmAb-968 | XmAb968 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Leukemia, Promyelocytic, Acute | Details |
| GR-1803 | GR-1803 | Phase 1 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Multiple Myeloma | Details |
| XmAb-18968 | XmAb-18968 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Leukemia, Myeloid, Acute | Details |
| SQZ-622(Novartis Pharma) | SQZ-622 | Phase 1 Clinical | Novartis Pharma Ag | Leukemia, Myeloid, Acute | Details |
| Forimtamig | RG-6234 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| RG-6232 | RG-6232 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Melanoma | Details |
| RG-6007 | RO-7283420; RG-6007 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Leukemia, Myeloid, Acute | Details |
| SAR-443216 | SAR-443216 | Phase 1 Clinical | Sanofi | Solid tumours; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms | Details |
| Recombinant oncolytic type II herpes simplex virus (Binhui Biopharmaceutical) | BS-006; oHSV2-BiTEs | Phase 1 Clinical | Wuhan Binhui Biotechnology Co Ltd | Solid tumours; Neoplasms; Melanoma; Uterine Cervical Neoplasms | Details |
| JNJ-78306358 | JNJ-78306358; JNJ-6358 | Phase 1 Clinical | Janssen Research & Development Llc | Solid tumours | Details |
| F-182112 | F-182112; F182112 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details |
| TNB-585 | TNB-585; AMG-340 | Phase 1 Clinical | Teneobio Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| BC-3448 | BC3448; BC-3448 | Phase 1 Clinical | Solid tumours | Details | |
| IBI-389 | IBI-389 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| hEGFRvIII-CD3 Bi-scFv | Phase 1 Clinical | Duke University | Glioblastoma; Glioma | Details | |
| JNJ-78278343 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| TQB-2825 | TQB-2825 | Phase 1 Clinical | Wuxi Biologics Co Ltd | Hematologic Neoplasms | Details |
| RGV-004 | RGV-004 | Phase 1 Clinical | Hangzhou Rongu Biotechnology Co Ltd | Lymphoma, B-Cell | Details |
| Recombinant humanized anti-BCMA/CD3 bispecific antibody(New Time Pharmaceutical) | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details | |
| NVG-111 | NVG-111 | Phase 1 Clinical | NovalGen Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Melanoma; Carcinoma, Non-Small-Cell Lung; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| BI-765049 | BI-765049; OBT-624 | Phase 1 Clinical | Oxford Biotherapeutics Ltd | Solid tumours | Details |
| GNC-039 | GNC-039 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis | Details | |
| RO-7425781 | RO-7425781 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| RO-7293583 | RO-7293583 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Skin Melanoma; Uveal melanoma; Melanoma | Details |
| Rezetamig | JNJ-8780; JNJ75348780; JNJ-75348780 | Phase 1 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Y-150(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | Y-150; Y150 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Multiple Myeloma | Details |
| EX-101 | EX-101; EX101 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| Plamotamab | XmAb-13676 | Phase 1 Clinical | Xencor Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| A-337 | A-337 | Phase 1 Clinical | Evive Biotech Ltd | Solid tumours; Neoplasms | Details |
| CCW-702 | CCW-702 | Phase 1 Clinical | The Scripps Research Institute Inc, Abbvie Inc | Prostatic Neoplasms | Details |
| Tepoditamab | MCLA-117 | Phase 1 Clinical | Pharmaceutical Research Associates, Institute Gustave-Roussy, Merus Nv, Vu University Medical Center, Lgc | Leukemia, Myeloid, Acute | Details |
| ERY-974 | ERY-974 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| InHeAb-01 | InHeAb-01; bsAB | Clinical | University Hospital Tuebingen | Neoplasms | Details |
| M-701 | M-701 | Phase 3 Clinical | Wuhan Yzy Biopharma Co Ltd | Ovarian Neoplasms; Solid tumours; Hydrothorax; Stomach Neoplasms; Neoplasms; Ascites; Colorectal Neoplasms; Pleural Effusion, Malignant; Carcinoma, Non-Small-Cell Lung | Details |
| ABBV-383 | TNB-383B; ABBV-383 | Phase 3 Clinical | Teneobio Inc, Abbvie Inc | Multiple Myeloma | Details |
| IMC-F106C | IMC-F106C; PRAME HLA-A02 | Phase 3 Clinical | Immunocore Ltd | Solid tumours; Melanoma | Details |
| Alnuctamab | CC-93269; EM-901; BMS-986349 | Phase 3 Clinical | Engmab Ag | Multiple Myeloma | Details |
| Catumaxomab | LP000 | Phase 3 Clinical | Trion Research, Neovii Biotech Gmbh | Ovarian Neoplasms; Stomach Neoplasms; Carcinoma; Neoplasms; Carcinoma, Ovarian Epithelial; Colonic Neoplasms; Urinary Bladder Neoplasms; Ascites; Breast Neoplasms | Details |
| Flotetuzumab | S-80880; MGD-006; RES-234 | Phase 2 Clinical | Macrogenics Inc | Leukemia, Myelogenous, Chronic; Hematologic Neoplasms; Leukemia; Leukemia, Hairy Cell; Mastocytosis, Systemic; Hodgkin Disease; Blastic Plasmacytoid Dendritic Cell Neoplasm; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Neoplasms, Plasma Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Leukemia, Myeloid, Acute; Leukemia, Biphenotypic, Acute | Details |
| Anti-CD3/humanized 3F8 bispecific antibody-activated T lymphocytes (University of Virginia) | Phase 2 Clinical | University Of Virginia | Neuroblastoma | Details | |
| Imvotamab | IGM-2323 | Phase 2 Clinical | Igm Biosciences Inc | Lymphoma, B-Cell, Marginal Zone; Myositis; Lupus Erythematosus, Cutaneous; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Arthritis; Lymphoma, Non-Hodgkin | Details |
| Foralumab | TZLS-401; NI-0401/α-CD3; NI-0401 | Phase 2 Clinical | Novimmune Sa, Bristol-Myers Squibb Company | Respiratory Tract Infections; Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Coronavirus Disease 2019 (COVID-19); Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Alzheimer Disease; Crohn Disease | Details |
| GNR-084 | GNR-084 | Phase 2 Clinical | Generium Pharmaceuticals, Iontas | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD3 and anti-HER2 BiTE-expressing T cell (MedImmune) | Phase 2 Clinical | University Of Michigan, Huazhong University Of Science And Technology, University Of Virginia Cancer Center, Medimmune | Breast Neoplasms | Details | |
| Vibecotamab | XmAb-14045 | Phase 2 Clinical | Xencor Inc | Leukemia, Myeloid; Myelodysplastic Syndromes; Blastic Plasmacytoid Dendritic Cell Neoplasm; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details |
| α/β CD3+/CD19+ cell depleted stem cell therapy (Mitchell Cairo) | Phase 2 Clinical | New York Medical College | Leukemia; Anemia; Thalassemia; Hodgkin Disease; Anemia, Aplastic; Thrombocytopenia; Lymphoma, Non-Hodgkin; Kostmann Syndrome; Anemia, Sickle Cell | Details | |
| MK-6070 | HPN-328; MK-6070 | Phase 2 Clinical | Harpoon Therapeutics | Small Cell Lung Carcinoma | Details |
| Cibisatamab | CEA-TCB; RG-7802; RO-6958688; CEA-CD3 TCB | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CD3/CD19 neg allogeneic BMT (National Institute of Allergy and Infectious Diseases/University of Pittsburgh) | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), University Of Pittsburgh | Primary Immunodeficiency Diseases; Female Urogenital Diseases; Inflammation | Details | |
| HPN-536 | HPN-536 | Phase 2 Clinical | Harpoon Therapeutics | Neoplasms | Details |
| MBS-303 | MBS-303 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| HBM-7022 | HBM-7022; AZD-5863 | Phase 2 Clinical | Harbour Biomed | Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Neoplasms; Digestive System Neoplasms; Esophageal adenocarcinoma; Carcinoma, Pancreatic Ductal; Gastrointestinal Neoplasms | Details |
| TAK-280 | MVC-280; TAK-280 | Phase 2 Clinical | Maverick Therapeutics Inc | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| GB-261 | GB-261 | Phase 2 Clinical | Genor Biopharma Co Ltd | Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| REGN-5459 | REGN-5459 | Phase 2 Clinical | Multiple Myeloma; Renal Insufficiency, Chronic | Details | |
| IBI-3003 | IBI3003; IBI-3003 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Multiple Myeloma | Details |
| MBS-314 | MBS-314 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Multiple Myeloma | Details |
| RO7515629 | RO7515629; RO-7515629; RG-6353 | Phase 2 Clinical | F. Hoffmann-La Roche Ag | Ovarian Neoplasms; Carcinoma, Renal Cell; Pancreatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BI-764532 | BI-764532; OBT620 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Small Cell Lung Carcinoma; Neoplasms; Neuroendocrine Tumors; Carcinoma, Neuroendocrine; Glioma | Details |
| GEN3017 | GEN-3017 | Phase 2 Clinical | Genmab A/S | Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| MP0533 | MP0533 | Phase 2 Clinical | Molecular Partners Ag | Leukemia, Myeloid, Acute | Details |
| CD30 biAb-AATC(The Medical College Of Wisconsin Nonprofit) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Leukemia; Hodgkin Disease; Lymphoma; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell, Cutaneous | Details | |
| APVO-436 | APVO-436; APVO436 | Phase 2 Clinical | Aptevo | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| Emfizatamab | GNC-038 | Phase 2 Clinical | SystImmune | Solid tumours; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Central Nervous System Lymphoma | Details |
| GEN-1047 | GEN-1047 | Phase 2 Clinical | Genmab A/S | Carcinoma, Non-Small-Cell Lung | Details |
| REGN-4336 | REGN-4336 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant | Details | |
| CM-350 | CM-350 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Solid tumours | Details |
| 1-A-46 | BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 | Phase 2 Clinical | Chengdu Chimagen Biosciences Co Ltd, BioRay Pharmaceutical Co Ltd | Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| LBL-034 | LBL-034 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms; Multiple Myeloma | Details |
| LBL-033 | LBL-033 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms | Details |
| CM-336(Connaught Biomedical Technology) | CM-336 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Multiple Myeloma | Details |
| ICP-B02 | CM-355; ICP-B02 | Phase 2 Clinical | Beijing Tiannuo Jiancheng Pharmaceutical Technology Co Ltd, Keymed Biosciences Co Ltd | Hematologic Neoplasms; Hematoma; Lymphoma; Lymphoma, Non-Hodgkin | Details |
| SMET-12 | SMET-12 | Phase 2 Clinical | Zhejiang Shimai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| QLS-31905 | QLS-31905 | Phase 2 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| Nebratamig | GNC-035 | Phase 2 Clinical | Solid tumours; Hematologic Neoplasms; Breast Neoplasms; Metastatic breast cancer; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| TAK-186 | EGFR x CD3 COBRA; MVC-101; TAK-186 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| EMB-06 | EMB-06; EMB06 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Multiple Myeloma | Details |
| AZD-0486 | TNB-486; AZD-0486; AZD0486 | Phase 2 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| BNT-142 | BNT-142 | Phase 2 Clinical | Biontech Se | Solid tumours | Details |
| CN-201 | CN-201 | Phase 2 Clinical | Curon Biopharmaceutical Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| ZG006 | ZG006; ZG-006 | Phase 2 Clinical | Gensun Biopharma Inc, Suzhou Zelgen Biopharmaceuticals Co Ltd | Solid tumours; Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Neuroendocrine | Details |
| EX-103 | EX-103; EX103 | Phase 2 Clinical | Guangzhou Excelmab Inc | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD3 monoclonal antibody (Jinan Tiankang Biological Products) | Phase 2 Clinical | Jinan Tiankang Biological Products Co Ltd | Anemia, Aplastic | Details | |
| Cevostamab | RG-6160; BFCR-4350A; RO-7187797 | Phase 2 Clinical | Genentech Inc | Multiple Myeloma | Details |
| Anti-CD3/anti-EGFR -activated T cells (Barbara Ann Karmanos Cancer Institute) | Phase 2 Clinical | Barbara Ann Karmanos Cancer Institute, University Of Virginia | Glioblastoma; Pancreatic Neoplasms | Details | |
| Ubamatamab | REGN-4018 | Phase 2 Clinical | Ovarian Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms | Details | |
| Anti-CD3-anti-HER2-activated T cells | Phase 2 Clinical | Transtarget | Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Prostatic Neoplasms | Details | |
| JCAR-014 | JCAR-014; JCAR021 | Phase 2 Clinical | Fred Hutchinson Cancer Research Center, Memorial Sloan Kettering Cancer Center, Seattle Children'S Research Institute, Juno Therapeutics Inc | Lymphoma, B-Cell; Leukemia; Leukemia, Lymphoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Candidiasis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| A-319 | A-319 | Phase 1 Clinical | Evive Biotech Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| BCMA TriTAC | HPN-217 | Phase 1 Clinical | Harpoon Therapeutics, Abbvie Inc | Multiple Myeloma | Details |
| Membrane bound ligand T-SIGn virus | NG-348 | Phase 1 Clinical | Akamis Bio Ltd | Neoplasms | Details |
| NG-641 | EnAd-FAP-BiTE; EnAd-FAP-Tac; NG-641; NG-aFAP | Phase 1 Clinical | Akamis Bio Ltd, University Of Oxford | Squamous Cell Carcinoma of Head and Neck; Neoplasms, Glandular and Epithelial; Neoplasm Metastasis | Details |
| Vixtimotamab | T-564; AMV-564 | Phase 1 Clinical | Amphivena Therapeutics Inc | Solid tumours; Myelodysplastic Syndromes | Details |
| MGD-014 | MGD-014 | Phase 1 Clinical | Macrogenics Inc | HIV Infections | Details |
| JNJ-63898081 | JNJ-8081; JNJ-63898081 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| K-193 | K-193 | Phase 1 Clinical | Beijing Lvzhu Biological Technology Co Ltd | Lymphoma, B-Cell | Details |
| Anti-EGFR-bispecific antibody armed activated T-cell therapy (Memorial Sloan Kettering Cancer Center) | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Pancreatic Neoplasms | Details | |
| Runimotamab | BTRC-4017A; RG-6194; RO-7227780 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| Autologous T-cell therapy (anti-PSMA-CD3), Roger Williams Medical Center | Phase 1 Clinical | Roger Williams Medical Center | Prostatic Neoplasms | Details | |
| ONO-4685 | ONO-4685 | Phase 1 Clinical | Merus Nv | Psoriasis; Lymphoma, T-Cell; Plaque psoriasis | Details |
| WVT-078(Novartis Pharma) | WVT-078 | Phase 1 Clinical | Novartis Pharma Ag | Multiple Myeloma | Details |
| M-802(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | M-802 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| Emirodatamab | AMG-427 | Phase 1 Clinical | Amgen Inc | Leukemia, Myeloid, Acute | Details |
| JNJ-63709178 | JNJ-9178; CNTO-9958; JNJ-63709178 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine, Genmab A/S | Leukemia, Myeloid, Acute | Details |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| CC-1 | CC-1 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Squamous Cell | Details |
| Acapatamab | AMG-160 | Phase 1 Clinical | Amgen Inc | Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| Pavurutamab | AMG-701 | Phase 1 Clinical | Amgen Inc | Multiple Myeloma | Details |
| Xaluritamig | AMG-509 | Phase 1 Clinical | Xencor Inc, Amgen Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| CC-3 | CC-3 | Phase 1 Clinical | Eberhard Karls University Of Tubingen, Germany | Colorectal Neoplasms; Gastrointestinal Neoplasms | Details |
| JS-203 | JS-203 | Phase 1 Clinical | Shanghai Junshi Biological Engineering Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| BC-004 | BC-004 | Phase 1 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Breast Neoplasms | Details |
| JY-016 | JY-016 | Phase 1 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Solid tumours; Colorectal Neoplasms; Lung Neoplasms | Details |
| EX-105 | EX-105; EX105 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| EMB-07 | EMB-07 | Phase 1 Clinical | Shanghai Epimab Biotherapeutics, Inc | Solid tumours; Ovarian Neoplasms; Stomach Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Adenocarcinoma of Lung; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Lymphoma; Uterine Neoplasms; Neoplasm Metastasis | Details |
| BA-3182 | BA-3182 | Phase 1 Clinical | Bioatla | Solid tumours; Adenocarcinoma | Details |
| BA-1202 | BA-1202 | Phase 1 Clinical | Solid tumours | Details | |
| CLN-978 | CLN-978 | Phase 1 Clinical | Adimab LLC | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| MGD-024 | MGD-024 | Phase 1 Clinical | Macrogenics Inc | Leukemia, Myelogenous, Chronic; Leukemia, Hairy Cell; Hodgkin Disease; Myelodysplastic Syndromes; Neoplasms; Blastic Plasmacytoid Dendritic Cell Neoplasm; Leukemia, B-Cell; Leukemia, Myeloid, Acute | Details |
| ARB-202 | ARB-202 | Phase 1 Clinical | Arbele Corp | Liver Neoplasms; Biliary Tract Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Esophageal adenocarcinoma; Gastrointestinal Neoplasms | Details |
| CX-904 | CX-904 | Phase 1 Clinical | Amgen Inc, Cytomx Therapeutics Inc | Solid tumours; Neoplasms | Details |
| CLN-049 | CLN-049 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| XmAb-819 | XmAb-819 | Phase 1 Clinical | Xencor Inc | Kidney Neoplasms; Carcinoma, Renal Cell | Details |
| JNJ-70218902 | JNJ-70218902 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| JNJ-67571244 | JNJ-67571244; JNJ-67371244; JNJ-1244 | Phase 1 Clinical | Johnson & Johnson | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| SCTB-35 | SCTB35; SCTB-35 | Phase 1 Clinical | SinoCelltech Ltd | Lymphoma, B-Cell | Details |
| JNJ-87890387 | JNJ-87890387 | Phase 1 Clinical | Janssen Research & Development Llc | Solid tumours | Details |
| XmAb-541 | XmAb541; XmAb-541 | Phase 1 Clinical | Xencor Inc | Ovarian Neoplasms; Ovarian germ cell tumor; Germinoma; Endometrial Neoplasms; Neoplasms, Germ Cell and Embryonal | Details |
| [89Zr]Zr-BI-764532 | Phase 1 Clinical | C.H. Boehringer Sohn Ag & Co. Kg, Boehringer Ingelheim Gmbh | Small Cell Lung Carcinoma; Carcinoma, Neuroendocrine | Details | |
| TGI-6 | TGI-6 | Phase 1 Clinical | Hefei TG ImmunoPharma Co Ltd | Solid tumours; Colorectal Neoplasms | Details |
| SIM-0500 | SIM-0500; SIM0500 | Phase 1 Clinical | Hainan Xiansheng Re Ming Pharmaceutical Co Ltd | Bone Marrow Neoplasms; Multiple Myeloma | Details |
| Recombinant anti BCMA/CD3 bispecific antibody(Hualan Genetic Engineering) | Phase 1 Clinical | Hualan Genetic Engineering (Henan) Co Ltd | Multiple Myeloma | Details | |
| Oncolytic Virus R130(Yunying Medical) | R-130-OV; R130; R-130 | Phase 1 Clinical | Shanghai Yunying Medical Technology Co Ltd | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Melanoma; Laryngeal Neoplasms; Pharyngeal Neoplasms; Lung Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Bone Neoplasms; Peritoneal Neoplasms; Sarcoma; Osteosarcoma; Head and Neck Neoplasms; Brain Neoplasms; Otorhinolaryngologic Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Nose Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma; Carcinoma, Bronchogenic; Liver Neoplasms; Ovarian Neoplasms; Kidney Neoplasms | Details |
| ASP2074 | ASP-2074; ASP2074 | Phase 1 Clinical | Astellas Pharma Global Development Inc | Solid tumours | Details |
| OBT620 | OBT620 | Phase 1 Clinical | Boehringer Ingelheim Gmbh, Oxford Biotherapeutics Ltd | Small Cell Lung Carcinoma | Details |
| CBA-1535 | CBA-1535 | Phase 1 Clinical | Chiome Bioscience Inc | Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Mesothelioma; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-008 | EGFR-TRACTr; JANX008; JANX-008 | Phase 1 Clinical | Janux Therapeutics Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-007 | JANX-007; PSMA-TRACTr | Phase 1 Clinical | Janux Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| TQB-2934 | TQB2934; TQB-2934 | Phase 1 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Multiple Myeloma | Details |
| JNJ-80948543 | JNJ-80948543 | Phase 1 Clinical | Janssen Research & Development Llc | Lymphoma, B-Cell; Neoplasms; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| PIT-565 | PIT-565 | Phase 1 Clinical | Novartis Pharma Ag | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Leukemia, Myeloid, Acute | Details |
| CC-312 | CC-312 | Phase 1 Clinical | CytoCares (Shanghai) Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ASP-2138 | ASP-2138 | Phase 1 Clinical | Astellas Pharma Global Development Inc, Xencor Inc | Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms | Details |
| ISB-2001 | ISB-2001 | Phase 1 Clinical | Ichnos Sciences Sa | Multiple Myeloma | Details |
| GR-1901 | GR1901; GR-1901 | Phase 1 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Leukemia, Myeloid, Acute | Details |
| AMG-794 | AMG-794 | Phase 1 Clinical | Amgen Inc | Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung | Details |
| QLS-31904 | QLS31904; QLS-31904 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours | Details |
| YK-012 | YK012 | Phase 1 Clinical | Lymphoma, B-Cell | Details | |
| RO-7428731 | RO-7428731; RG-6156 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Glioblastoma | Details |
| GBR-1342 | GBR-1342; ISB-1342 | Phase 1 Clinical | Glenmark Pharmaceuticals Ltd | Multiple Myeloma | Details |
| Recombinant humanized anti-CD19/CD3 bispecific antibody(New Time Pharmaceutical) | LNF-1904 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| XmAb-968 | XmAb968 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Leukemia, Promyelocytic, Acute | Details |
| GR-1803 | GR-1803 | Phase 1 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Multiple Myeloma | Details |
| XmAb-18968 | XmAb-18968 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Leukemia, Myeloid, Acute | Details |
| SQZ-622(Novartis Pharma) | SQZ-622 | Phase 1 Clinical | Novartis Pharma Ag | Leukemia, Myeloid, Acute | Details |
| Forimtamig | RG-6234 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| RG-6232 | RG-6232 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Melanoma | Details |
| RG-6007 | RO-7283420; RG-6007 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Leukemia, Myeloid, Acute | Details |
| SAR-443216 | SAR-443216 | Phase 1 Clinical | Sanofi | Solid tumours; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms | Details |
| Recombinant oncolytic type II herpes simplex virus (Binhui Biopharmaceutical) | BS-006; oHSV2-BiTEs | Phase 1 Clinical | Wuhan Binhui Biotechnology Co Ltd | Solid tumours; Neoplasms; Melanoma; Uterine Cervical Neoplasms | Details |
| JNJ-78306358 | JNJ-78306358; JNJ-6358 | Phase 1 Clinical | Janssen Research & Development Llc | Solid tumours | Details |
| F-182112 | F-182112; F182112 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details |
| TNB-585 | TNB-585; AMG-340 | Phase 1 Clinical | Teneobio Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| BC-3448 | BC3448; BC-3448 | Phase 1 Clinical | Solid tumours | Details | |
| IBI-389 | IBI-389 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| hEGFRvIII-CD3 Bi-scFv | Phase 1 Clinical | Duke University | Glioblastoma; Glioma | Details | |
| JNJ-78278343 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| TQB-2825 | TQB-2825 | Phase 1 Clinical | Wuxi Biologics Co Ltd | Hematologic Neoplasms | Details |
| RGV-004 | RGV-004 | Phase 1 Clinical | Hangzhou Rongu Biotechnology Co Ltd | Lymphoma, B-Cell | Details |
| Recombinant humanized anti-BCMA/CD3 bispecific antibody(New Time Pharmaceutical) | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details | |
| NVG-111 | NVG-111 | Phase 1 Clinical | NovalGen Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Melanoma; Carcinoma, Non-Small-Cell Lung; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| BI-765049 | BI-765049; OBT-624 | Phase 1 Clinical | Oxford Biotherapeutics Ltd | Solid tumours | Details |
| GNC-039 | GNC-039 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis | Details | |
| RO-7425781 | RO-7425781 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| RO-7293583 | RO-7293583 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Skin Melanoma; Uveal melanoma; Melanoma | Details |
| Rezetamig | JNJ-8780; JNJ75348780; JNJ-75348780 | Phase 1 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Y-150(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | Y-150; Y150 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Multiple Myeloma | Details |
| EX-101 | EX-101; EX101 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| Plamotamab | XmAb-13676 | Phase 1 Clinical | Xencor Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| A-337 | A-337 | Phase 1 Clinical | Evive Biotech Ltd | Solid tumours; Neoplasms | Details |
| CCW-702 | CCW-702 | Phase 1 Clinical | The Scripps Research Institute Inc, Abbvie Inc | Prostatic Neoplasms | Details |
| Tepoditamab | MCLA-117 | Phase 1 Clinical | Pharmaceutical Research Associates, Institute Gustave-Roussy, Merus Nv, Vu University Medical Center, Lgc | Leukemia, Myeloid, Acute | Details |
| ERY-974 | ERY-974 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| InHeAb-01 | InHeAb-01; bsAB | Clinical | University Hospital Tuebingen | Neoplasms | Details |
This web search service is supported by Google Inc.
